Hello All,
I have been using the example fe55.C which is available in the garfieldpp/Examples/Heed directory for checking the amount of primary ionisation that a particular photon can produce for different gas mixtures. I have used 2 photon energies: 5900 eV and 8000 eV , and two gas mixtures: Ar / 5% CH4, and Ne / 5% CH4 at 1 atm and 293.15 K. Further, I have reduced the dimensions of the cylinder inside which the x-rays can ionise the gas mixture.
Original dimensions in fe55.C Example:
diameter = 7.8
length = 10.
The dimensions I am using:
diameter = 0.1
length = 0.45
Hence, because of the low dimensions that I am using, it is expected for most of the photons to escape without interacting at all. Hence, while filling the histogram, an if condition of if (ne > 0) hElectrons.Fill(ne); is used by me.
The reason for using low dimensions is to observe escape peaks, that is when K-alpha X-rays of Ar or Ne can escape from the medium if an inner electron is extracted from their atoms because of the incident radiation.
The W-values of Ne / 5% CH4 and Ar / 5% CH4 given by HEED are: 34.995 eV and 26.4256 eV respectively.
Hence, now that we know the W-values, we can expect beforehand what should be the difference in the number of primary electrons in the primary peak and secondary peak for the mixtures, knowing the K-alpha X-ray energy values of Neon and Argon. From hyperphysics, the values for Neon and Argon taken by me are 848.61 eV and 2957.682 eV.
Hence,
Expected difference for Argon mixture: K-alpha energy / W-value = 2957.682 / 26.4256 which is around 110
Expected difference for Neon mixture: 848.61 / 34.995 which is around 25
The results from HEED for Ar / 5% CH4 seem perfectly as expected, that is there is a difference of about 110 primary electrons between the primary peak and the secondary (escape) peak, but I can’t understand the results for Ne / 5% CH4. For 5.9 KeV photons, the secondary peak is somewhere I wouldn’t expect based on my calculations, and for 8 KeV photons, the expected primary peak becomes the secondary peak which is a bigger mystery to me. So my question is that are there some physics processes that I am missing here?
Here are the attached 1D histograms showing number of Primary electrons obtained across multiple number of independent events:
-
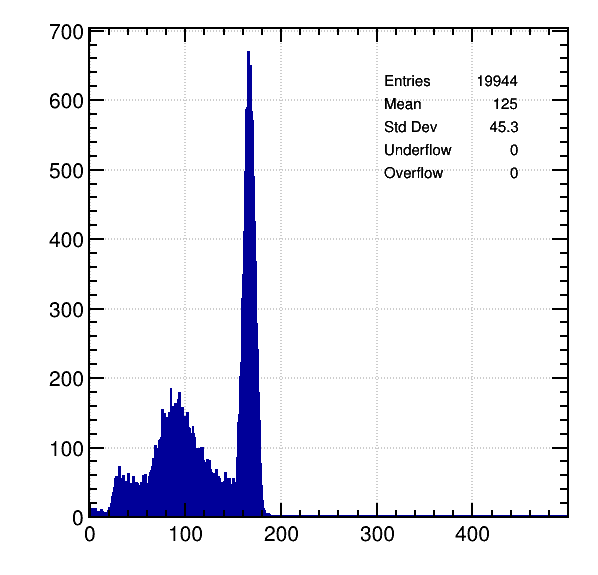
Ne / 5% CH4, 5.9 KeV:
It is seen that difference between primary electrons for both peaks is more than 25. I can’t understand why.
-
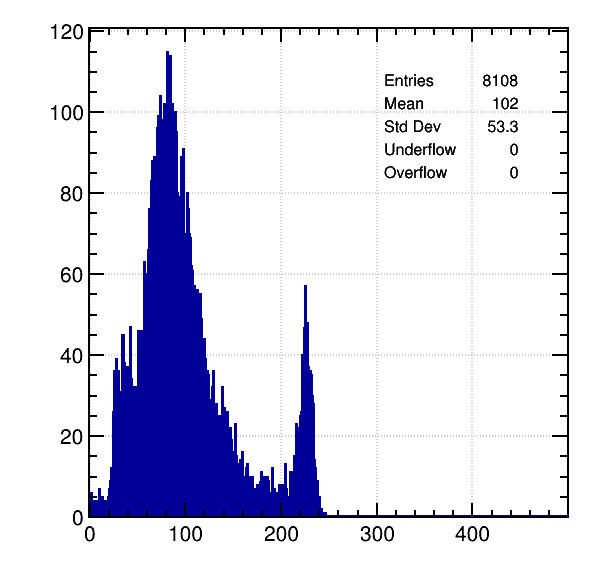
Ne / 5% CH4, 8 KeV:
Here, the expected primary peak becomes secondary. Again, I can’t understand the physics behind this.
-
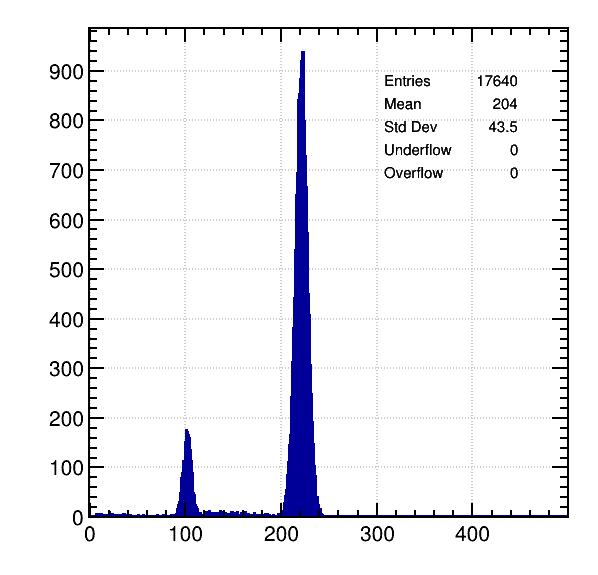
Ar / 5% CH4, 5.9 KeV:
Perfect match with calculations: Difference of about 110 primary electrons between both peaks.
-
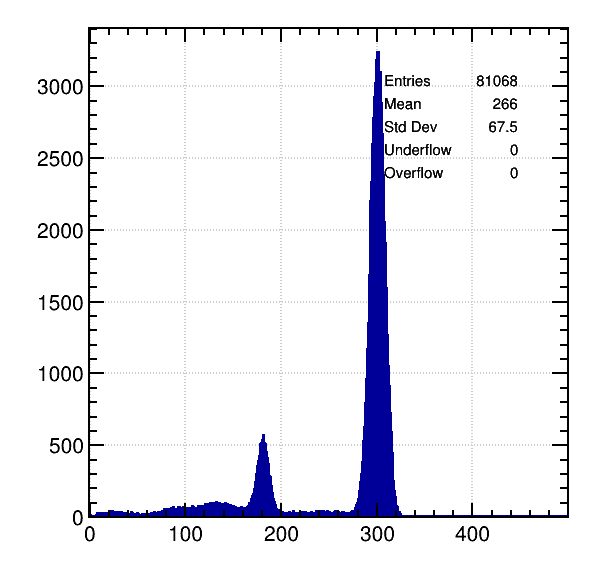
Ar / 5% CH4, 8 KeV:
Again a perfect match with calculations: Difference of about 110 primary electrons between both peaks.
Attached code:
fe55.C (2.7 KB)
In summary, my question is- why do the number of primary electrons produced by the X-rays in Ne / 5% CH4 show a sort of behaviour which I cannot predict, when the results for Ar / 5% CH4 seem to be perfectly as per my predictions. Are there some physical processes that I am not taking into account in case of Ne / 5% CH4 mixture?
Thank you.